Further Resources on the Science of Psychedelics and Ketamine
Image: https://nushama.com/post/ketamine-vs-classical-psychedelics/
Current scientific and research based have demonstrated how psychedelic medicine has the restorative and curative effects on awakening, healing, and expanding the mind and consciousness. Psychedelic medicines, like Psilocybin and MDMA, are now in Phase 3 trials for treatment of depression in Cancer Patients, PTSD in Veterans, Treatment resistant depression, and many more ongoing studies at John Hopkins, Univ. California San Francisco, New York University, and most Importantly at MAPS(Multidisciplinary Association for Psychedelic Assisted Therapies) -international and national sites.
Plant medicines and soon to be approved medicines, Psilocybin and MDMA have been and are being used in all parts of the world and cultures for centuries in healing and transcendental practices.
FAQs on Ketamine
To understand what this means and the remarkable effect of ketamine on anxiety and depression, let’s start with how the neurons in your brain work.
What effect does ketamine have on the neurons, the fundamental cells of the brain, in the treatment of anxiety and depression?
The Role Neuron Circuitry of the Brain
The neuron has a cell body that contains the nucleus. The nucleus is where DNA is stored and also where the cell processes of the neuron is regulated. The Neuron also contains other cell body: ribosomes-needed to make proteins like neurotransmitters and mitochondria-the miniature energy producing power plants of the cell.
Extending off the cell body of the neuron are axons. The axons are long extensions of the cell body that at it's end junction communicate to other dendrites, extensions of other neurons, The communications is maintained by nerve electrical signals and neurotransmitters that transmit messages from one neuron to another. Dendrites also branch off at the other end of the neuron and form clusters of nerve fibers that send and recieve messages from other cell bodies.
In the most developed part of the human brain, the cerebral cortex there are an estimated 14-16 neurons,making connections, firing signals, and forming pathways that are required in executive functioning, learning, memory, speech, and emotions etc.
What is the role of Neurotransmitter in the Brain?
Electric impulses, (Action Potentials) are transmitted from the axon of one neuron to the dendrites of another neuron. The junction where the neurons communicate is called the synapse. Neurotransmitters are brain chemicals, like serotonin, Nor-epinephrine, Dopamine and others. To transmit the nerve impulse neurotransmitters travel across the gap or synpatic cleft between neurons as chemical messengers to allow the impulse or signal to be propagated.
Neurotransmitters are made in the neurons and stored in tiny sacs, vesicles, at the ends of axons. The cell membrane or outer covering of the neurons are covered with receptors, which channel the neurotransmitters through to the next neuron. There receptors are specific for each type of neurotransmitter. The neurotransmitter fit like a key on the receptor which then opens the channel into the cell.
The nerve impulse at the end of the tip, also affect the opening of Calcium channels. Thes Calcium-charged elements then cause the vesicles to attach to the neuron cell membrane, which then allows for the pouring of Neurotransmitters to be secreted into the synapse. Neurotransmitters diffuse across the synapse gap and bind to their matching and specific receptors in the membrane of the postsynaptic neuron (the target neuron). The binding opens the channels leading into the cell interior and calcium, sodium, magnesium, and other ions flow in.
The flow of incoming ions such as calcium and sodium depolarizes( or changes the charge) the cell membrane, which gives the inside of the cell to be more negatively charges. When the depolarization threshold is reached the neuron fires an electrical signal. This impulse is then transmitted through the cell, down its axon, and on to the next neuron in the chain.
After the neurotransmitter has done its jobs in the synapse, it is taken back up or (reuptake) into the pre-synaptic cell membrane. From there the neurotransmitter is taken back into the cell and stored once again in the tiny sacs, vesicles. Neurotransmitters now repackaged await the signal of another cycle of impulses to repeat the actions as the messenger between neurons once again.
When the neurotransmitters are re-absorbed, the synapse turns off. The entire process of impulse and neurotransmitter communication back to reuptake into the cell occurs in milliseconds and happens billions of time a day, 24 hours a day!
Types of Neurotransmitters
Different neurotransmitters affect neurons in different ways. Some neurotransmitters excite other neurons, and some neurotransmitters inhibit or turn off other neurons with their signaling. Dopamine, which is also a key neurotransmitters can excite and inhibit neurons to which it communicates.
In the brain, the neurotransmitters glutamate and dopamine are excitatory. The inhibitory neurotransmitters are GABA (gamma-aminobutyric acid), serotonin, and dopamine.
The release, removal, and reuptake of neurotransmitters is tightly regulated. If there is an effect on the reuptake of neurotransmitters, the concentration neurotransmitter or the sensitivity of neuron's impulse, there can be a disruption in the brain's communication, connectivity, and balance. This can lead to a dysregulated state of either too much excitation or inhibition in specific parts of the brain.
Glutamate is the most common excitatory neurotransmitter in the brain. It plays a particularly important role in neuroplasticity (the brain’s ability to form new synapses and neural connections over a lifetime), learning, and forming memories. When there’s too much glutamate in the brain, the postsynaptic neurons can become hyperexcited; when there’s way too much glutamate in the brain, it can damage neurons or even cause neuron death.
What is the Effect of Stress on the Brain, Neuronal Glutamate Circuits, and Damaged Neurons?
Toxic level and persistent stress affects the neurons of the cortex. Toxic stress puts the brain in an inflammatory and increases cortisol concentrations in the brain. Cortisol can cause neurons atrophy or early death and shrinkage of neurons. Dendrites also decrease in their ability to branch and spread, which leads to fewer connections between neurons. The axons are thinner and smaller. Glutamate signaling is also less effective and responsive.
It has been shown that in chronically depressed people, the size of the prefrontal cortex is smaller and connections to other key components of the brain, are restricted and become dysfunctional in the emotional (limbic) and memory centers (hippocampus) of the brain.
The toxic and inflammatory state of the brain, in trauma, depression, anxiety limits effectiveness of the glutamate neuronal pathways.
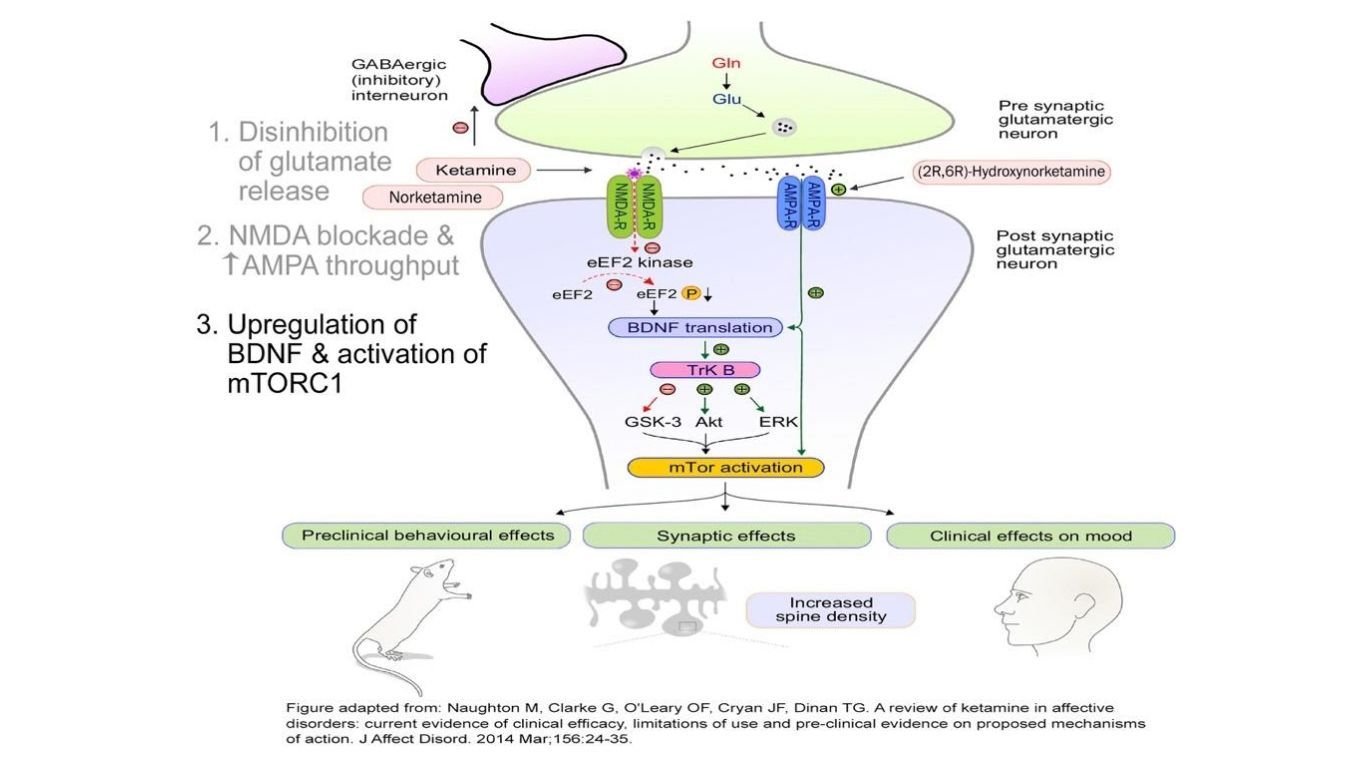
What is the Role of Ketamine on the Glutamate processes in depression, anxiety, trauma and stress?
Ketamine, which is an anesthetic works by activating glutamate release into the synapse. Researchers studying depression discovered that glutamate receptors can be "dysfunctional" in people with depression. -this is focus of ketamines effect on the glutamate system in treating depression. Ketamine when used as an anesthetic, was also found to lift symptoms of depression. Since the 1990's more research has shown that small doses of ketamine when given to severely depressed patients, who did not respond to standard antidepressants like SSRI's had remarkable results.
How does Ketamine improves Neuroplasticity in Brain?
Not only does Ketamine increase glutamate transmission and a net increase in AMPA activation, it also allows for quick up-regulation of neuronal production and release of BDNF (brain-derived neurotrophic factor). BDNF is a growth factor in the brain that promotes neuronal growth and enhances survival it leads to restoration and neuroplasticity of neurons and their connectivity.
In addition, Ketamine works on another important pathway called mTOR(mammalian target of rapamycin) which is involved in regulation of cell growth and synthesis of proteins necessary for long-term memory. The combination of mTOR and BDNF also improve connectivity in the synpses of the prefrontal cortex and hippocapmus- areas involved in processing emotional regulation, memory. Research has shown that within a hours of treatment doses of Ketamine, there is repair, regrowth, and enhanced connectivity in areas of the brain, PFC and hippocampus, damaged by toxic and or persistent stress. When this type of neuroplasticity occurs, symptoms of trauma, anxiety, and depression are diminished.
It is also, important to realize that at higher doses(Anesthetic level doses) of Ketamine, neuroplasticity and increase in BDNF does not occur.
How does Ketamine activates Glutamate receptors selectively?
We know that ketamine does affect other receptors but it most important receptor it activates are glutamate receptors.
Neurons have many binding sites for glutamate, but when it comes to ketamine, two are of particular interest: the NMDA (N-methyl-D-aspartate) receptor and the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor.
Glutamate activates the ion channels in both NMDA (N-methyl-D-aspartate) and AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors.
At the very low dose, Ketamine increases release of glutamate from presynaptic cleft into the synapse, which has an antidepressant effect. However, Ketamine then preferentially blocks glutamate at the NMDA receptors of the postsynaptic cell but not at the adjacent AMPA receptors. This causes a net effect to increase AMPA activation. In addition, Ketamine triggers the neuron to make more AMPA receptors, moving them into the membrane of the synapse area.
Ketamine improves other mechanisms in the neural processes as well?
Further research has uncovered the effect of Ketamine on other receptors, NMDA and parvalumin interneurons. Ketamine blocks one part of the NMDA receptors, and in these parvalbumin interneurons, the effect is and increase activity of brain circuitry which awakens the brain. Ketamine can trigger increased release of the neuromodulators dopamine and noradrenaline; it also binds weakly to nicotinic and opioid receptors. More actions of Ketamine are also known to effect on organelles (structures) in the neuron that are involved in signaling, protein and lipid synthesis, and transport proteins.
Ketamine and Enhances Connectivity
Toxic level stress impacts the brain at both micro -cellular and macro structural level on the brain. Chronic depression, anxiety, and PTSD leads to decreased size, response, and connectivity in the key parts of the brain. The prefrontal cortex is where a significant portion of executive functioning ocurs, planning, organizing, self regulation, problem solving. Ketamine enhances global connectivity within the prefrontal cortex and the pathways in other sub-regions of the brain.
The Science of Ketamine
Ketamine is a breakthrough medicine of the mind. It can rapidly—often very quickly—lift the symptoms of anxiety, depression, PTSD, OCD, and other conditions. For most people, the action of a single small dose of ketamine lasts for a week and possibly longer. With a science-backed regimen of repeated and scheduled doses, the effects of improving mood, depression, anxiety, and inner knowing can extend for weeks to months. In addition, the subjective effects on consciousness and the psyche often lead to profound emotional and psychological insights.
Ketamine, a Schedule III medication used in assisted psychotherapy, is an off-label treatment for various chronic treatment-resistant mental health conditions. Medical professionals have used this medication as an analgesic and anesthetic agent since the 1970's. Today, it helps treat alcohol addiction, depression, PTSD, substance dependencies, and other psychiatric diagnoses.